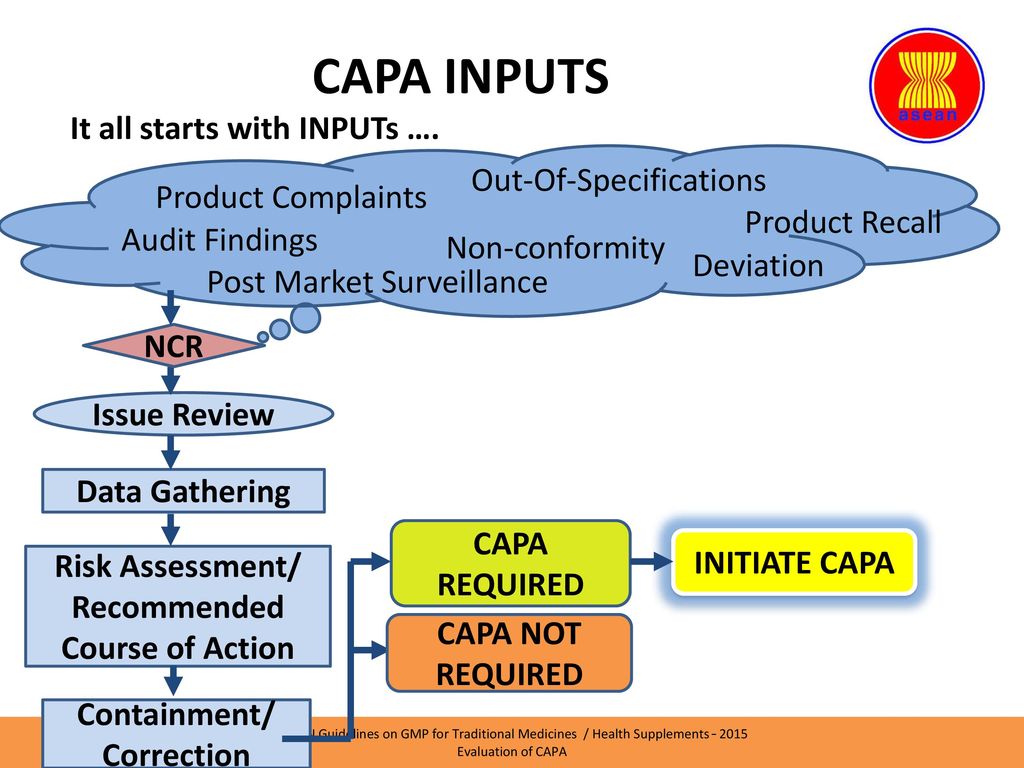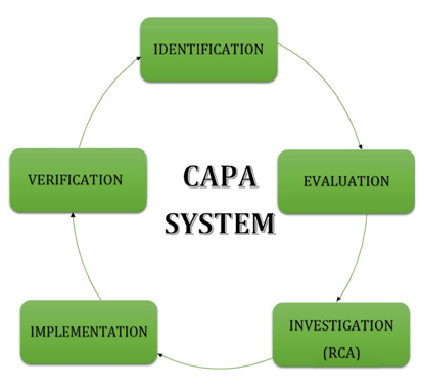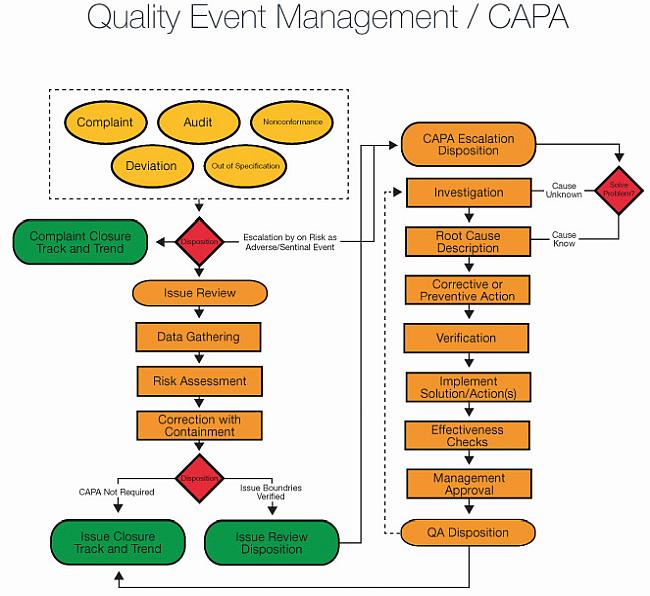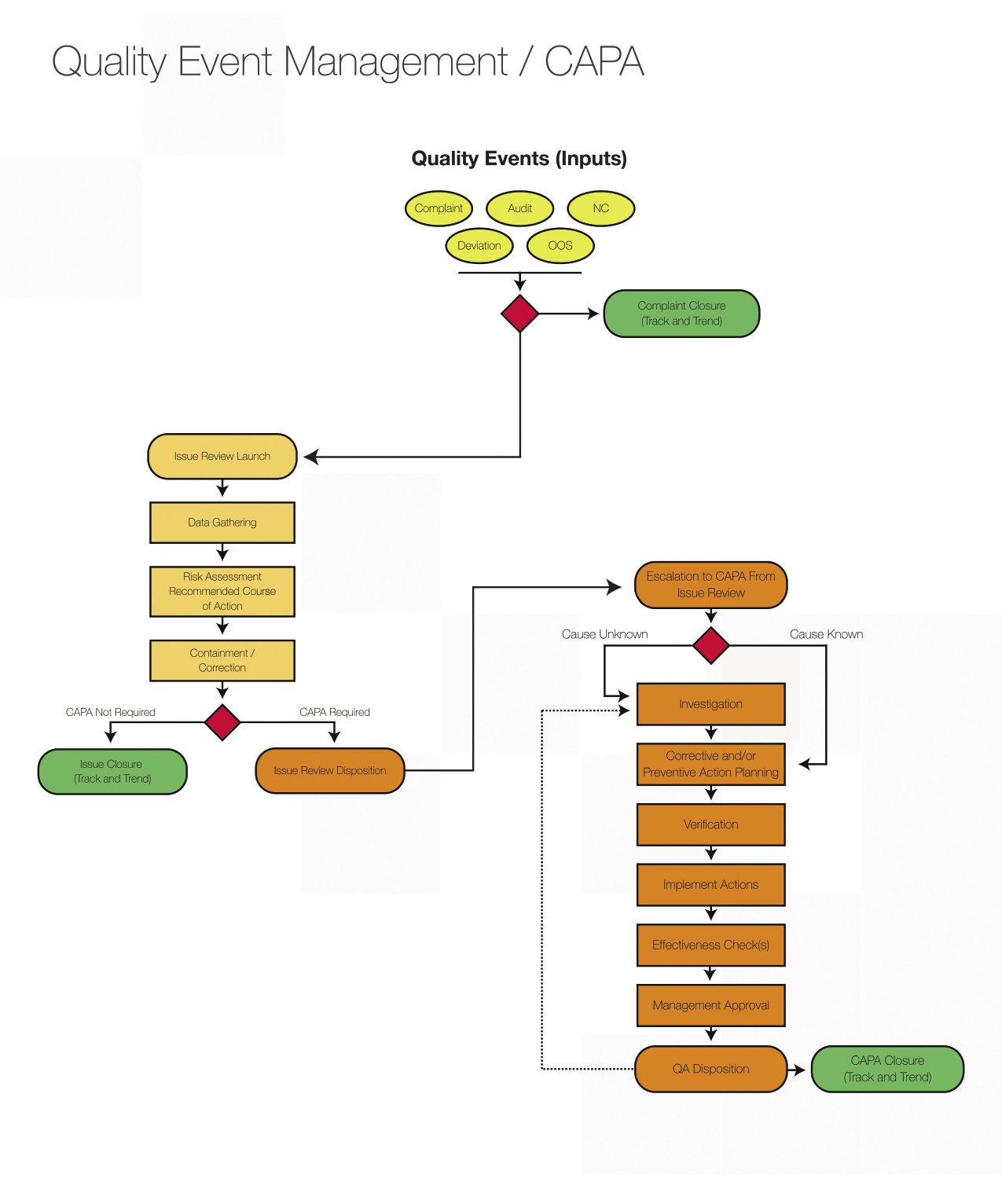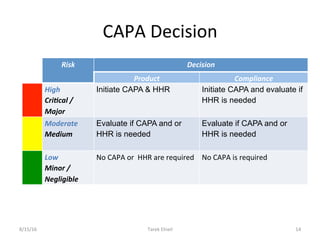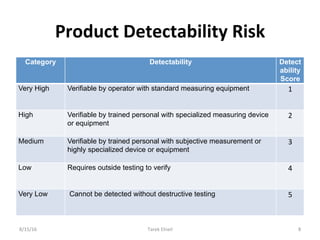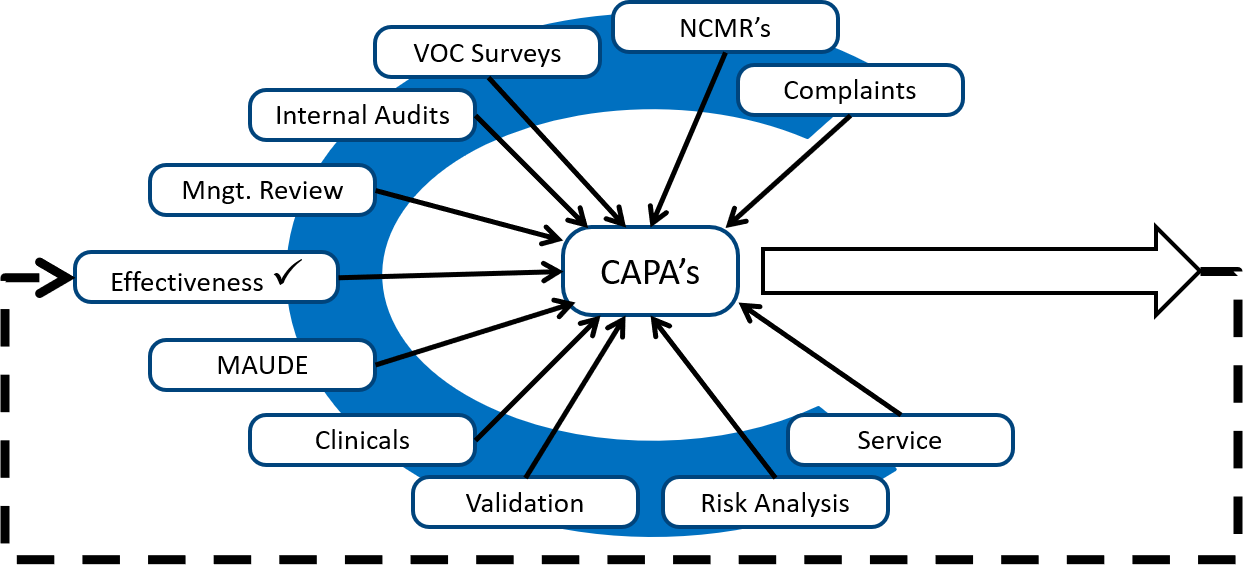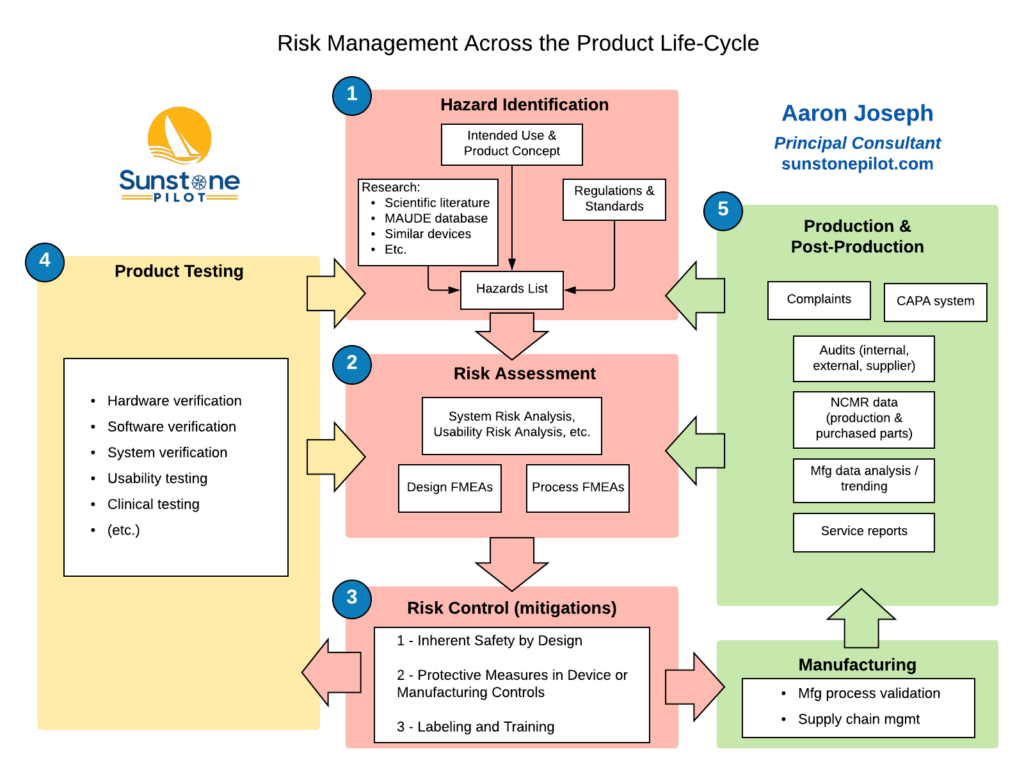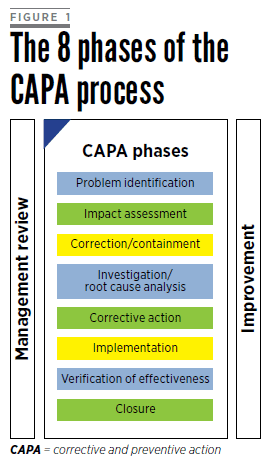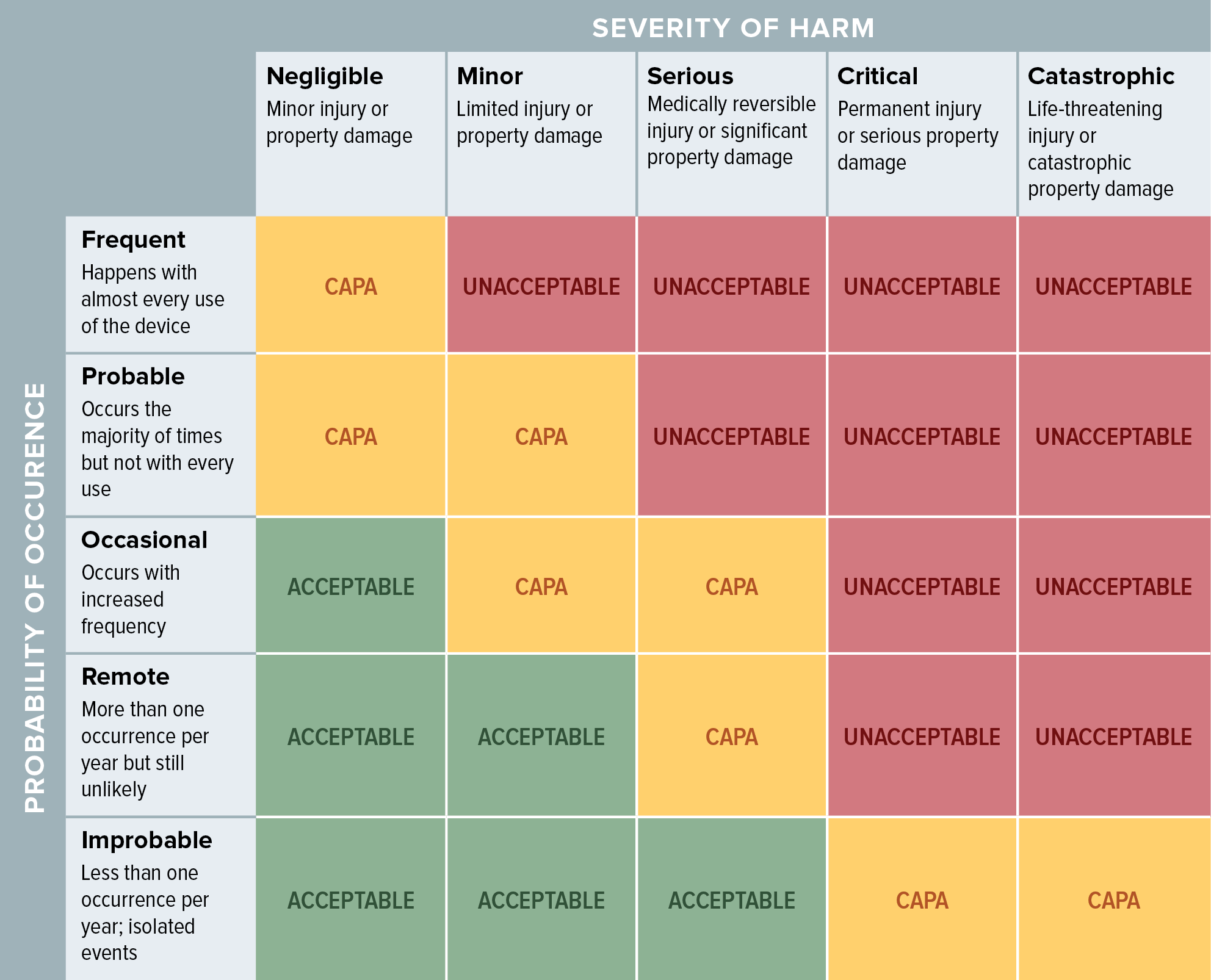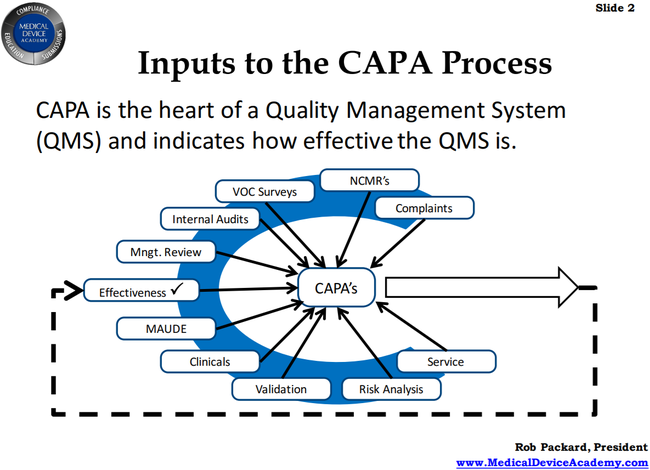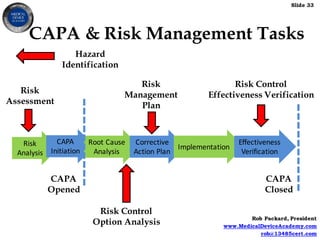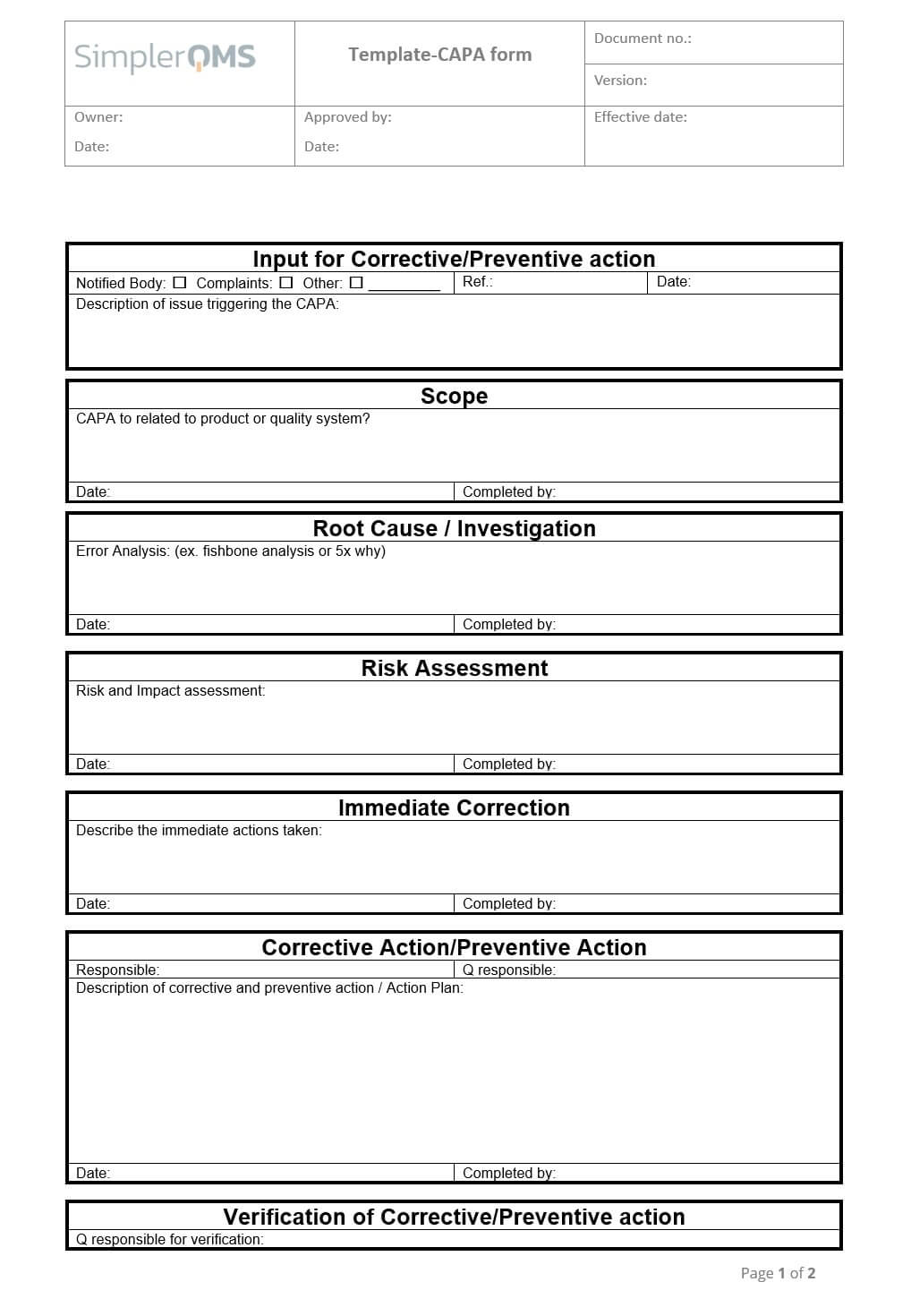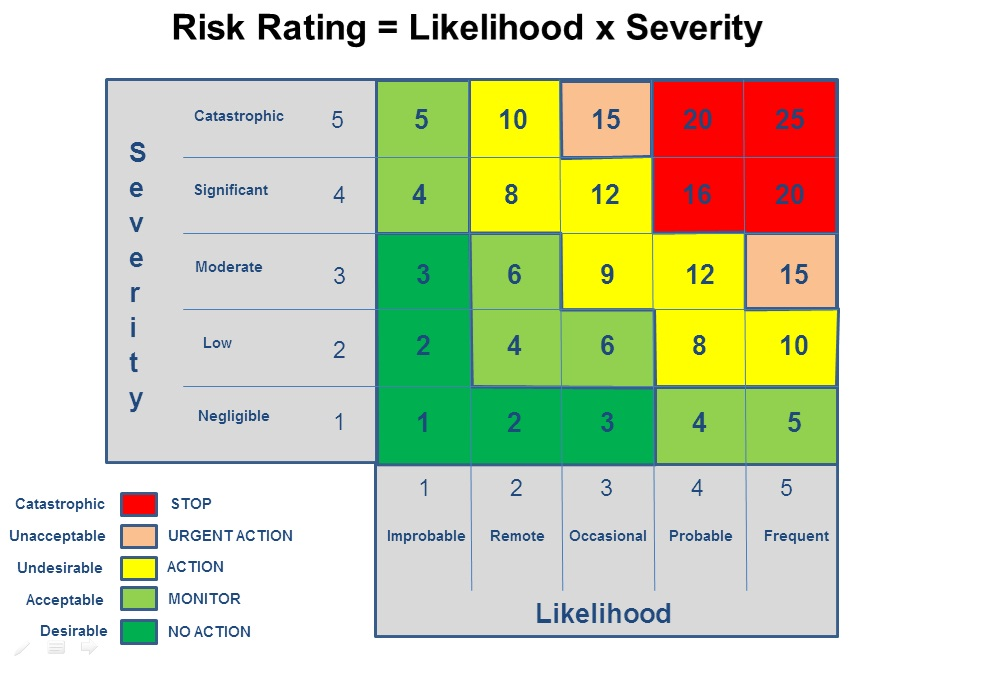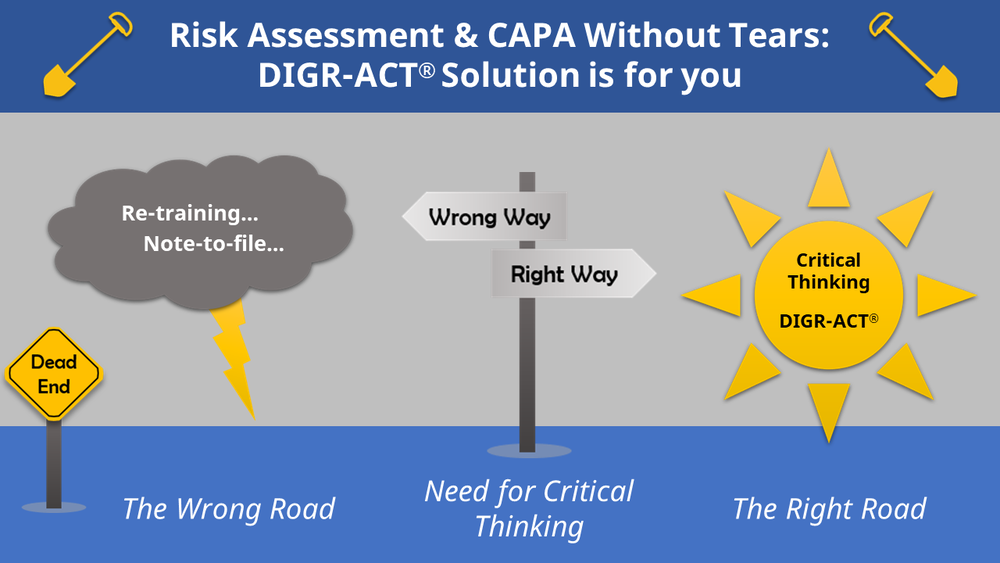
Live Webinar: Risk Assessment & CAPA without Tears: The DIGR-ACT® Critical Thinking Solution — Clinical Pathways

Use of risk management throughout the product life cycle. Abbreviations... | Download Scientific Diagram

Effective Risk Based CAPA Management Strategies and Continuous Improvement. by Compliance Global Inc - Issuu